Alzheimer’s Disease
Alzheimer’s Disease (AD) is progressive, neurodegenerative disease. It is most common in people ages 60-85, and the risk increases significantly as age increases. Memory impairment is typically the first symptom of AD, while problems with thinking, decision-making, simple task execution, and motor movements increase as the disease progresses(1,2). Symptoms of AD are caused by amyloid plaques and tau aggregates, which result in brain tissue inflammation, disrupted synapses, and nerve cell degeneration(3). Recent research suggests that decreased oxygen levels in the brain (due to decreased blood flow) causes amyloid plaque formation and the associated brain tissue damage(4, 5). Current AD treatments only target symptoms of AD and include cholinesterase inhibitors and N-methyl D-aspartate (NMDA) antagonists(6). However these classes of medications can have many adverse side effects and do not treat the rood cause of AD(7,8).
Extivita Therapies for Alzheimer’s Disease:
Extivita Therapies for Alzheimer’s Disease:

Hyperbaric Oxygen Therapy

Neurofeedback

Supplements

Nutritional IV Therapy

Pulsed Electromagnetic Field Therapy
Hyperbaric Oxygen Therapy for Alzheimer’s Disease:

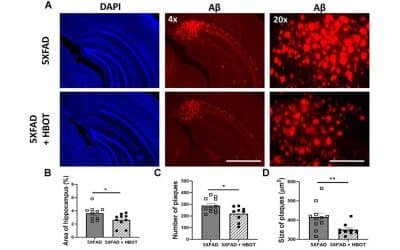
HBOT may have benefits for Alzheimer’s Disease (AD) because it directly affects the underlying causes of AD while maintaining a low risk of potential side effects(9). HBOT significantly increases blood flow and oxygen concentration in the brain, which are two of the potential underlying causes of AD(10, 11). In animal models of AD, HBOT has been shown to decrease the formation, size, and number of amyloid plaques as well as reduce the formation of tau aggregates(12).
In addition, HBOT decreases neuroinflammation, a main issue in AD(12, 13). The effects of HBOT on blood flow, oxygen levels, amyloid plaques, and inflammation in the brain suggest its potential as a low-risk treatment for AD.
Effects of HBOT on Alzheimer’s Disease:

Decreased Inflammation

New Blood Vessel Formation
Increased Stem Cell Activity

Neurofeedback for Alzheimer’s Disease:
Neurofeedback (NF) has the potential to improve the cognitive decline associated with Alzheimer’s Disease (AD). Electroencephalograms (EEG’s), which measure brainwave activity, have found that patients with AD have decreased alpha activity and increased delta and theta activity(14). Increasing peak alpha frequency has been shown to improve cognitive processing speed and executive function (but not memory) in older individuals who experience cognitive decline like that in AD(15). Decreasing theta amplitude has been shown to improve memory and stabilize cognitive function in aging populations as well as patients with AD(16). Therefore, research suggests that increasing alpha activity and decreasing theta activity may reduce the cognitive decline associated with AD.
IV Therapy for Alzheimer’s Disease:
We strongly recommend our NAD+ IV Trio for reducing the risk of Alzheimer’s disease (AD). The NAD+ IV Trio includes an NAD+ IV, Myer’s Cocktail IV, and glutathione IV, all of which work together to improve cellular issues characteristic of AD. NAD+ levels are known to decrease with age, and its deficiency is implicated in various neurodegenerative disorders including AD(17). Interest in NAD+ as a potential treatment for AD continues to grow due to NAD+‘s ability to reduce Aβ plaques, improve mitochondrial dysfunction, and reduce brain cell death(18-20).
In addition to NAD+, the Myer’s IV has strong antioxidant effects that may reduce risk of AD by decreasing oxidative stress and neuroinflammation(21-22). Levels of magnesium, which is also in the Myer’s IV, are low in many people with AD and may contribute to AD progression and cognitive decline(23).
Last, but not least, increasing glutathione levels may improve cognitive function and reduce risk of AD(24-26). Glutathione is considered the “master antioxidant”, but its levels are low in the brains of people with AD(24). Glutathione deficiency results in increased oxidative stress and associated cellular damage, so increasing its levels is vital to reducing risk of AD(26). Taken together, NAD+, the Myer’s IV, and glutathione target many root causes of AD as well as improve numerous cellular functions that are impaired in AD. Glutathione also has strong potential to decrease the oxidative stress heavily involved in AD.

Pulsed Electromagnetic Field Therapy for Alzheimer’s Disease:
Pulsed electromagnetic frequency (PEMF) therapy may counteract some of the harmful underlying causes of Alzheimer’s disease (AD). It is known that people with both AD and mild cognitive decline (which can lead to AD) have decreased blood flow (hypoperfusion) in certain regions of the brain(4, 27). Hypoperfusion results in decreased oxygen levels in the brain, an issue that has been implicated in the progression of AD(4, 5). PEMF can increase microcirculation in the brain, therefore combating hypoperfusion and low oxygen levels that likely contribute to AD(28).
News & Research for Alzheimer’s Disease:
A Review of the Application of Hyperbaric Oxygen Therapy in Alzheimer’s Disease
Abstract Alzheimer's disease (AD) is considered as the most common cause of dementia in elderly population. While the exact mechanism of AD has not been discovered, hyperbolic [hyperbaric] oxygen therapy (HBOT) has been proven to be effective in the treatment of this...
Alzheimer’s disease: hyperbaric oxygen proposed as treatment in new study
Alzheimer’s disease, the most common form of dementia, has long been associated with a build-up of plaques (clumps of protein) in the brain. Scientists in Israel have shown that a type of oxygen therapy can stop new plaques forming and even remove existing plaques in...
Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer’s disease mouse model and in elderly patients
Abstract Vascular dysfunction is entwined with aging and in the pathogenesis of Alzheimer's disease (AD) and contributes to reduced cerebral blood flow (CBF) and consequently, hypoxia. Hyperbaric oxygen therapy (HBOT) is in clinical use for a wide range of medical...
References
- “Alzheimers Disease” 2019. https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/symptoms-causes/syc-20350447.
- “Stages of Alzheimer’s.” Alzheimer’s Disease and Dementia. Accessed August 7, 2019. https://alz.org/alzheimers-dementia/stages.
- Selkoe, Dennis J, and John Hardy. “The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years.” EMBO Molecular Medicine 8, no. 6 (June 2016): 595–608. https://doi.org/10.15252/emmm.201606210.
- Nishimura, Tsunehiko, et al. “Decreased Cerebral Blood Flow and Prognosis of Alzheimer’s Disease: A Multicenter HMPAO-SPECT Study.” Annals of Nuclear Medicine, vol. 21, no. 1, Jan. 2007, pp. 15–23. Springer Link, doi:10.1007/BF03033995.
- Zhang, Feng, et al. “Impacts of Acute Hypoxia on Alzheimer’s Disease-Like Pathologies in APPswe/PS1dE9 Mice and Their Wild Type Littermates.” Frontiers in Neuroscience, vol. 12, May 2018. PubMed Central, doi:10.3389/fnins.2018.00314.
- “How Is Alzheimer’s Disease Treated?” National Institute on Aging. Accessed August 8, 2019. https://www.nia.nih.gov/health/how-alzheimers-disease-treated.
- Singh, Ravneet, and Nazia M. Sadiq. “Cholinesterase Inhibitors.” StatPearls, StatPearls Publishing, 2020. PubMed, http://www.ncbi.nlm.nih.gov/books/NBK544336/.
- Ali, Thibault B., et al. “Adverse Effects of Cholinesterase Inhibitors in Dementia, According to the Pharmacovigilance Databases of the United-States and Canada.” PLoS ONE, vol. 10, no. 12, Dec. 2015. PubMed Central, doi:10.1371/journal.pone.0144337.
- Heyboer, Marvin, et al. “Hyperbaric Oxygen Therapy: Side Effects Defined and Quantified.” Advances in Wound Care, vol. 6, no. 6, June 2017, pp. 210–24. PubMed Central, doi:10.1089/wound.2016.0718.
- Tal, Sigal, et al. “Hyperbaric Oxygen May Induce Angiogenesis in Patients Suffering from Prolonged Post-Concussion Syndrome Due to Traumatic Brain Injury.” Restorative Neurology and Neuroscience, vol. 33, no. 6, IOS Press, Jan. 2015, pp. 943–51. content.iospress.com, doi:10.3233/RNN-150585.
- Choudhury, Ryan. “Hypoxia and Hyperbaric Oxygen Therapy: A Review.” International Journal of General Medicine, vol. Volume 11, Nov. 2018, pp. 431–42. ResearchGate, doi:10.2147/IJGM.S172460.
- Shapira, Ronit, Beka Solomon, Shai Efrati, Dan Frenkel, and Uri Ashery. “Hyperbaric Oxygen Therapy Ameliorates Pathophysiology of 3xTg-AD Mouse Model by Attenuating Neuroinflammation.” Neurobiology of Aging 62 (2018): 105–19. https://doi.org/10.1016/j.neurobiolaging.2017.10.007.
- Thom, Stephen R. “Hyperbaric Oxygen – Its Mechanisms and Efficacy.” Plastic and Reconstructive Surgery, vol. 127, no. Suppl 1, Jan. 2011, pp. 131S-141S. PubMed Central, doi:10.1097/PRS.0b013e3181fbe2bf.
- Lizio, Roberta, et al. “Electroencephalographic Rhythms in Alzheimer’s Disease.” International Journal of Alzheimer’s Disease, vol. 2011, Hindawi, 12 May 2011, p. e927573, doi:https://doi.org/10.4061/2011/927573.
- Angelakis, Efthymios, et al. “EEG Neurofeedback: A Brief Overview and an Example of Peak Alpha Frequency Training for Cognitive Enhancement in the Elderly.” The Clinical Neuropsychologist, vol. 21, no. 1, Jan. 2007, pp. 110–29. PubMed, doi:10.1080/13854040600744839.
- Luijmes, Robin E., et al. “The Effectiveness of Neurofeedback on Cognitive Functioning in Patients with Alzheimer’s Disease: Preliminary Results.” Neurophysiologie Clinique/Clinical Neurophysiology, vol. 46, no. 3, June 2016, pp. 179–87. ScienceDirect, doi:10.1016/j.neucli.2016.05.069.
- Johnson, Sean, and Shin-ichiro Imai. “NAD + Biosynthesis, Aging, and Disease.” F1000Research, vol. 7, Feb. 2018. PubMed Central, doi:10.12688/f1000research.12120.1.
- Gong, Bing, et al. “Nicotinamide Riboside Restores Cognition through an Upregulation of Proliferator-Activated Receptor-γ Coactivator 1α Regulated β-Secretase 1 Degradation and Mitochondrial Gene Expression in Alzheimer’s Mouse Models.” Neurobiology of Aging, vol. 34, no. 6, June 2013, pp. 1581–88. ScienceDirect, doi:10.1016/j.neurobiolaging.2012.12.005.
- Long, Aaron N., et al. “Effect of Nicotinamide Mononucleotide on Brain Mitochondrial Respiratory Deficits in an Alzheimer’s Disease-Relevant Murine Model.” BMC Neurology, vol. 15, no. 1, Mar. 2015, p. 19. BioMed Central, doi:10.1186/s12883-015-0272-x
- Hou, Yujun, et al. “NAD+ Supplementation Normalizes Key Alzheimer’s Features and DNA Damage Responses in a New AD Mouse Model with Introduced DNA Repair Deficiency.” Proceedings of the National Academy of Sciences, vol. 115, no. 8, National Academy of Sciences, Feb. 2018, pp. E1876–85. www.pnas.org, doi:10.1073/pnas.1718819115.
- Yao, Yuemang et al. “Brain inflammation and oxidative stress in a transgenic mouse model of Alzheimer-like brain amyloidosis.” Journal of neuroinflammation vol. 1,1 21. 22 Oct. 2004, doi:10.1186/1742-2094-1-21
- Heo, Jae-Hyeok, et al. “The Possible Role of Antioxidant Vitamin C in Alzheimer’s Disease Treatment and Prevention:” American Journal of Alzheimer’s Disease & Other Dementias®, SAGE PublicationsSage CA: Los Angeles, CA, Jan. 2013. Sage CA: Los Angeles, CA, journals.sagepub.com, doi:10.1177/1533317512473193.
- Chui, Dehau, et al. “Magnesium in Alzheimer’s Disease.” Magnesium in the Central Nervous System, edited by Robert Vink and Mihai Nechifor, University of Adelaide Press, 2011. PubMed, http://www.ncbi.nlm.nih.gov/books/NBK507256/.
- Mandal, Pravat K., et al. “Brain Glutathione Levels–a Novel Biomarker for Mild Cognitive Impairment and Alzheimer’s Disease.” Biological Psychiatry, vol. 78, no. 10, Nov. 2015, pp. 702–10. PubMed, doi:10.1016/j.biopsych.2015.04.005.
- Mandal, Pravat K., et al. “Cognitive Improvement with Glutathione Supplement in Alzheimer’s Disease: A Way Forward.” Journal of Alzheimer’s Disease, vol. 68, no. 2, IOS Press, Jan. 2019, pp. 531–35. content.iospress.com, doi:10.3233/JAD-181054.
- Pocernich, Chava B., and D. Allan Butterfield. “Elevation of Glutathione as a Therapeutic Strategy in Alzheimer Disease.” Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease, vol. 1822, no. 5, May 2012, pp. 625–30. ScienceDirect, doi:10.1016/j.bbadis.2011.10.003.
- Sierra-Marcos, Alba. “Regional Cerebral Blood Flow in Mild Cognitive Impairment and Alzheimer’s Disease Measured with Arterial Spin Labeling Magnetic Resonance Imaging.” International Journal of Alzheimer’s Disease, vol. 2017, Hindawi, 1 Mar. 2017, p. e5479597, doi:https://doi.org/10.1155/2017/5479597.
- Bragin, Denis E., et al. “Increases in Microvascular Perfusion and Tissue Oxygenation via Pulsed Electromagnetic Fields in the Healthy Rat Brain.” Journal of Neurosurgery, vol. 122, no. 5, May 2015, pp. 1239–47. PubMed Central, doi:10.3171/2014.8.JNS132083.