Post-COVID Recovery: Healing with HBOT
Post-COVID challenges can leave many grappling with unresolved health issues. Hyperbaric Oxygen Therapy (HBOT) is a medical treatment that enhances the body’s natural healing process by delivering oxygen at higher-than-atmospheric pressures. Post-COVID challenges can leave many grappling with unresolved health issues. This increased oxygen availability promotes cellular repair, reduces inflammation, and boosts immune function. These benefits have shown promising results in various conditions, including the persistent and often debilitating symptoms of post-COVID.
Long term outcomes of hyperbaric oxygen therapy in post covid condition: longitudinal follow-up of a randomized controlled trial
Coronavirus disease 2019 (Covid-19) represents a current pandemic disease caused by a novel severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) leading to formidable global effects. Long COVID, also known as post covid-19 condition, encompasses a range of symptoms that persist for weeks or months after the acute phase of infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The occurrence of long COVID spans a wide spectrum, afflicting 10–30% of non-hospitalized cases, 50–70% of those who required hospitalization, and 10–12% of vaccinated cases1–3. The suggested pathogenesis of long COVID involves a multitude of mechanisms including immune system dysregulation, exaggerated inflammatory response, hyperviscosity and microvascular damage, with more complex consequences in organs with high oxygen demand such as the central nervous system and the heart4. Numerous studies have explored the description, occurrence, and long-term consequences of the syndrome. However, a limited number of these studies have delved into interventions designed to ameliorate the core pathology and enhance clinical symptoms. Among these, the most promising treatment intervention, validated in well-controlled randomized controlled trials, has demonstrated the ability to induce notable neuroplasticity and substantially enhance clinical outcomes, involves the utilization of a novel hyperbaric oxygen therapy (HBOT) protocol5,6. The clinical benefits of HBOT were assessed within a timeframe of 1–3 weeks after the conclusion of the last HBOT sessions, while investigations into its long-term consequences have yet to be undertaken5.
The existing treatment options for long COVID are primarily informed by small-scale pilot studies within the context of long COVID or have drawn from successful approaches in managing other conditions7, such as the use of β-blockers for addressing postural orthostatic tachycardia syndrome (POTS)7, low-dose naltrexone for mitigating neuroinflammation8, intravenous immunoglobulin to address immune dysfunction9, tailored dietary approaches, and cognitive-behavioral therapy (CBT). However, the majority of these possibilities necessitate rigorous clinical testing, and expedited clinical trials are imperative10.
In recent years, a mounting body of evidence has emerged concerning the neuroplasticity-inducing effects of the new protocols of hyperbaric oxygen therapy (HBOT)11–18. HBOT entails the application of elevated atmospheric pressure in combination with increased oxygen levels, which enhances the diffusion of oxygen to poorly perfused tissues. The new protocol of HBOT, using the hyperoxic-hypoxic paradox (HHP), is one of the first therapeutic intervention already in clinical use today for the specific goal of inducing the regeneration of damaged brain tissue11,12,17. HHP is a newly suggested paradigm aiming to enhance the endogenous repair mechanisms, while providing an optimal microenvironment11. These effects include stem cells stimulation, migration and differentiation, mitochondrial proliferation/biogenesis, mitochondrial transfer and angiogenesis11,12,17. In our randomized controlled trial, we reported that HBOT can improve both cognitive, psychiatric, fatigue, sleep and pain symptoms in patients of long COVID5. However, one of the major limitations was that results were measured 1–3 weeks after the last HBOT sessions.
The aim of the current study was to evaluate the long term effects of HBOT on patients suffering from post-COVID-19 who participated in the randomized controlled trial.
Results
Patients’ baseline characteristics demographics, and high-risk comorbidities, are detailed in Table Table1.1. No statistically significant differences in baseline characteristics were observed between the original study’s HBOT group and the current cohort with long term evaluation.
In the context of quality of life assessed by the SF-36 questionnaire, the initial, short -term, evaluation post HBOT revealed substantial and statistically significant enhancements across most domains, except physical functioning as denoted in Table Table2.2. These improvements were characterized by moderate to large effect sizes, indicative of notable clinical impact. Upon protracted assessment, there was an improvement in the physical functioning domain (10.32 ± 21.62, p2 = 0.014), which did not reach statistical significance following correction for multiple comparisons (p2 corrected = 0.111). Other domains did not exhibit statistically significant score ameliorations, as outlined in Table Table2.2. Refer to Fig. 1 for the general health score. Notably, the long-term effect size pertinent to physical functioning exhibited a moderate magnitude, further enhancing the comparatively mild effect size observed 1–3 weeks post HBOT (ES1 = 0.59 vs ES2 = 0.16). A large effect size in both the physical limitations and pain domains were noted at the long term evaluation, compared to the moderate one post HBOT (ES1 = 1.08 vs ES2 = 10.73 and ES1 = 0.86 vs ES2 = 0.62, respectively, Table Table2.2).
Transitioning to the sleep evaluation conducted by the PSQI questionnaire, the short-term evaluation following HBOT underscored notable improvements in various facets, including the global score, sleep quality, sleep latency, sleep disturbances, and daytime dysfunction (p1 < 0.001). These improvements were accompanied by effect sizes of moderate magnitude (ES1 = 0.47–0.79, Table Table22).
The improvements at the end of the HBOT sessions, in those 5 sleep domains, were persistent (p2 = 1, Fig. 2). The global PSQI short term medium effect size improved to a large effect size (ES2 = 0.94 vs ES1 = 0.79). All other improved domains moderate effect sizes persisted in the long-term follow-up. Time from the last HBOT session was not a significant covariate for all domains.
In the context of neuropsychiatric symptoms as evaluated by the BSI-18 short-term evaluation subsequent to HBOT, a noteworthy increment was observed in the total score ((− 8.93 ± 10.74, p1 < 0.001) accompanied by a large effect size of 0.81 (Fig. 3). This augmentation extended to specific domains encompassing somatization, depression, and anxiety, each displaying statistically significant improvements (p1 < 0.01) coupled with effect sizes of moderate magnitude (0.88, 0.58, and 0.51, respectively). The beneficial effect of HBOT was persistent, without significant different for the effect achieved at the end of the HBOT in both total and sub-domain scores (p2 > 0.5). It is pertinent to note that the long-term effect sizes mirrored those observed during the short-term evaluation.
In addition, both pain severity (− 0.97 ± 1.39, p1 < 0.001, ES1 = 0.69) and pain interference (− 1.96 ± 2.33, p1 < 0.001, ES1 = 0.83) exhibited significant changes during the short-term assessment post HBOT. In the long-term evaluation, there was non-significant further improvement within these domains (p2 = 0.89 and 0.42). The effect sizes for pain severity (ES2 = 0.6 vs ES1 = 0.69) and pain interference (ES2 = 0.95 vs ES1 = 0.83) remained consistently moderate and large respectively, reinforcing their stability over time.
Time from the last HBOT session was not a significant covariate for all domains in all questionnaires (Table S1).
Discussion
In this long-term longitudinal follow-up of the active treatment group in our original randomized controlled trial, we found HBOT effects on quality of life, emotional well-being, sleep quality and neuropsychological symptoms in long COVID patients were maintained even more than 1 year (486 ± 73) after the last HBOT session. In all quality of life domains (SF-36), emotional domains (BSI-18), sleep quality (PSQI) domains pain severity and interference, long term scores were not statistically different from the beneficial effects evaluated 1–3 weeks after HBOT. The persisted improvements, more than a year after the last HBOT session, further support the growing knowledge that the new protocols of HBOT induced repairment and neuroplasticity at the biological level and those the clinical effect is not transient.
A recent meta-analysis has showed more than 57% of long covid syndrome patients continue to suffer from symptoms more than 12 months after infection. These main symptoms include frailty, physical limitations, fatigue, and cognitive deficits19. The current long term evaluation confirms that the new protocols of HBOT has an effective lasting effect on long covid patients more than 1 year after their treatment.
Long COVID has been linked to persistent psychiatric symptoms, such as depression, anxiety, and somatization20. Benedetti et al., identified alterations in brain microstructure, specifically in the superior and posterior corona radiata, superior longitudinal fasciculus, and cingulum, using MRI Diffusion Tensor Imaging (DTI) measures21. Additionally, COVID can induce significant perfusion changes in both insula, hippocampus, putamen, prefrontal and cingulate cortex22–24, areas associated with pain severity and pathological pain interference. In brain functional and structural connectivity analysis of our original study we have found that HBOT improved disruptions in white matter tracts and alters the functional connectivity organization of neural pathways attributed to cognitive and emotional recovery in post-COVID-19 patients6. This correlation between improvements in psychiatric symptoms and MRI-detected changes underscores the biological underpinnings of this condition and the therapeutic impact of HBOT. The finding that the clinical results were preserved after one year, reenforcing the previous findings that these are permanent changes driven by constant microstructural changes, i.e. brain injury recovery.
The pathogenesis of long COVID within the central nervous system involves a multitude of mechanisms4. One potential factor is the disruption of the blood–brain barrier (BBB) induced by the cytokine storm, leading to neuroinflammation and neuronal injury. Another possible contributor is the direct neurotropism of SARS-CoV-225. An exaggerated inflammatory response, with elevated levels of TGF-β, has been proposed as a mechanism for the development of neuropsychiatric and other neurological disorders in COVID-1926.
Furthermore, the neurological involvement in COVID-19 might be associated with the development of demyelination disorders, as previous coronavirus infections have been linked to neurodegeneration and demyelination27. Notably, the high expression of Angiotensin converting enzyme 2 (ACE2) in specific brain regions, such as the substantia nigra and the limbic system, could increase the interaction between SARS-CoV-2 and neurons, potentially leading to neurological complications28,29. The dysfunction of GABA-ergic neurons, due to inflammation, and high circulating ACE2 blocking direct activation of pre-sympathetic neurons, have been linked with chronic fatigue and dysexecutive syndrome long covid30,31. Lastly, hypervisocity-hypoperfusion syndrome associated with thrombotic events resulting in ischemic incidents, hypoxia, mitochondrial dysfunction,and metabolic dysfunction have also been suggested32–35.
These various pathways collectively culminate in dysfunctional brain tissue or chronic brain injury. New HBOT protocols have been recently shown to facilitate neuroplasticity and enhance brain injury recovery, even when administered months or years after the initial injury12. These protocols, including the one implemented in our original and present study, leverage the “Hyperoxic-Hypoxic paradox” (HHP). This paradox involves the repeated fluctuations in pressure and oxygen concentrations, which, in turn, trigger the expression of genes and activate metabolic pathways vital for regeneration. Remarkably, this is achieved without exposing the brain to perilous hypoxic conditions11,13,36. At the subcellular level, HBOT restores mitochondria function (in both neurons and glia cells) and metabolism37, attenuated by long COVID. By delivering high oxygen concentrations, HBOT can enhance oxygen delivery to tissues, reversing the local hypoxia and aiding in recovery of injured tissue38. At the cellular level, HBOT anti-inflammatory effects modulates the release of cytokines and inflammation associated with long COVID. By stimulating vasculogenic stem cells, HBOT induces angiogenesis39, addressing the vascular damage or thrombosis caused by long COVID. Lastly, HBOT has the ability to facilitate neurogenesis within compromised brain tissue12–15,17. It is plausible to suggest that the combination of these effects could serve as the underlying mechanisms contributing to the observed permanent clinical improvements associated with HBOT, as corroborated by the results of our present study (Fig. 4).
Numerous clinical studies have explored the impact of HBOT on neurodegenerative diseases40, as well as on other neurological conditions such as stroke and TBI, which may contribute to the development of neurodegenerative diseases like Alzheimer’s disease (AD)40. Evidence indicates that HBOT induced neuroplasticity with significant improvements in motor and cognitive function among stroke survivors17,41,42. HBOT treatment has demonstrated enhancements in both cognitive function and quality of life for chronic TBI patients41. Considering that age is a major risk factor for several neurodegenerative diseases, it is crucial to examine the effects of HBOT on the neurobiology of aging. HBOT has shown efficacy in ameliorating age-related cognitive deficits in healthy elderly subjects18. Moreover, HBOT exhibits a high safety profile in elderly adults, with barotrauma and visual changes being the primary side effects. Less than a handful of studies have evaluated the long-term effects of HBOT in any neurological indication and brain injury in specifics. Weaver et al. reported improved questionnaire based scores in traumatic brain injury patients post HBOT and 6 months later, while improvements regressed 12 months after HBOT43. However, this study did not utilize one of the newer HHP based protocols. Our group has recently reported the long-term effects of such a protocol on military veterans suffering from post-traumatic stress disorder (PTSD) who were treated with HBOT. Similarly, two years post the last HBOT session, the clinical effects were persistent and not attenuated44. As mentioned above, these protocols targets injured tissue recovery and true neuroplasticity, which will enable long term clinical effects.
The study has several limitations. First, the sample size was relatively small with 31 patients in total. Second, the primary endpoint in the original study, cognitive function, as well as brain imaging were not evaluated in the current longitudinal evaluation. Third, in the original RCT, patients who received sham intervention were not evaluated long term. Since the original sham group, after completing the study protocol, were offered to be treated with HBOT, and most of them received it (27/39, 69%) they could not serve as a proper control group for the current study.
In conclusion, HBOT can improve the quality of life, quality of sleep, psychiatric and pain symptoms of patients suffering from long COVID. The clinical improvements gained by HBOT are persistent even 1 year after the last HBOT session.
Cited By: PubMed Central
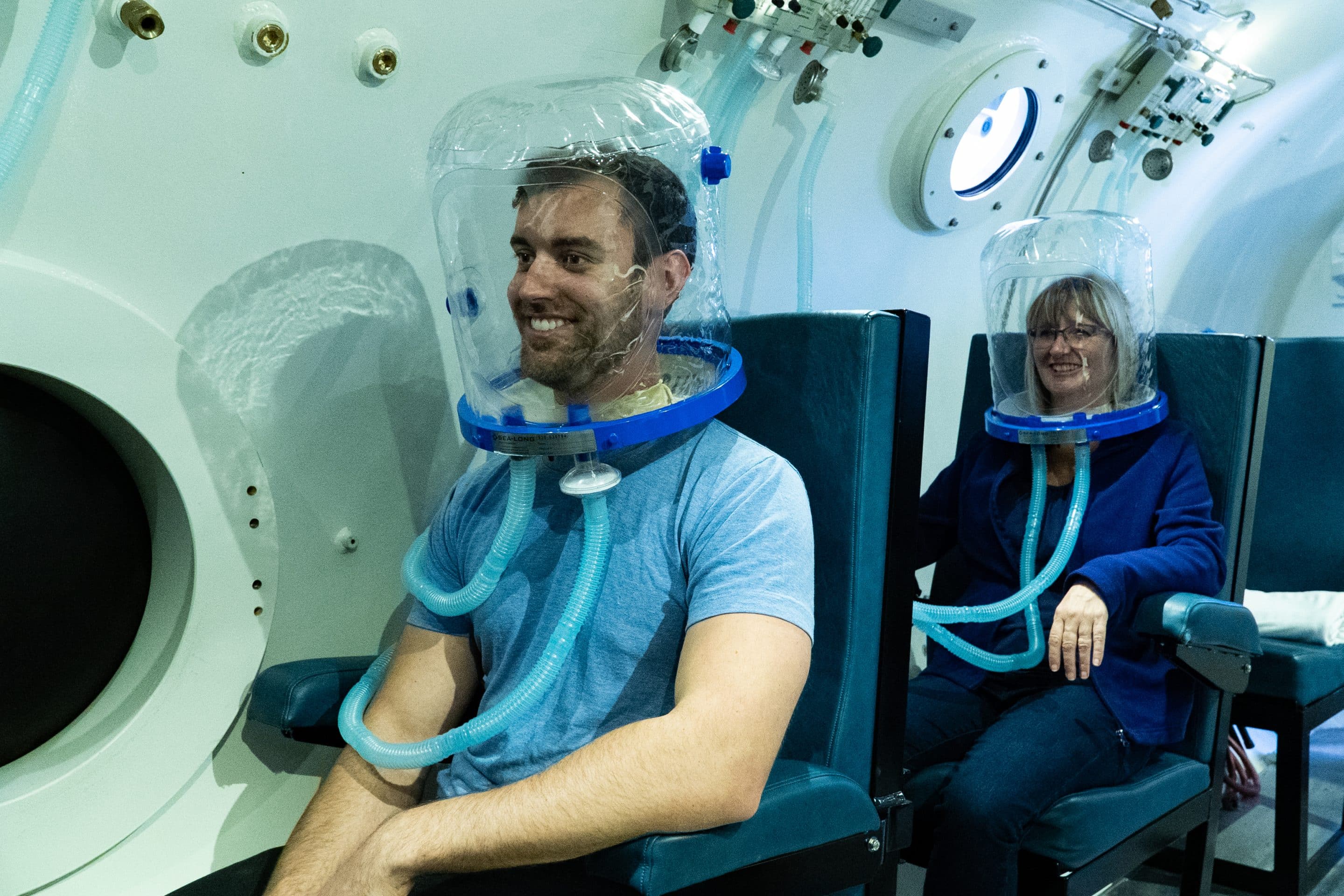
If you or someone you know is struggling with the lingering effects of post-COVID, Extivita, is here to support your recovery journey. We offer two state-of-the-art multiperson chambers, where each session lasts an hour and a half, with treatment at 2.0 ATA—equivalent to 33 feet below sea level. This precise pressure level enhances the therapeutic benefits of the treatment.
During your session, you can relax in comfort with tablets available at each seat, allowing you to stream your favorite shows. Additionally, a trained and certified hyperbaric technician will be inside the chamber with you, ready to assist and ensure your safety throughout the treatment.
Located in the heart of the Research Triangle Park (RTP) area in North Carolina, Extivita is easily accessible for those seeking advanced treatments. In addition to HBOT, we offer complimentary services such as nutritional IV therapy, which can further aid recovery for COVID long haulers by replenishing essential nutrients and boosting your overall wellness.
Contact us today to learn more about how our comprehensive approach at Extivita can help you regain your health and vitality.
Read More

How Extivita Integrative Therapies Support Restful, Restorative Sleep
Getting consistent, high-quality sleep is one of the most important foundations of overall health and well-being. Yet, stress, inflammation, and environmental factors often disrupt natural sleep patterns, leaving many people feeling fatigued and unrefreshed. At...

Extivita Expands to Jacksonville, NC—Bringing Life-Changing Therapies to the Coast
Jacksonville, NC – Extivita, North Carolina’s leading center for Hyperbaric Oxygen Therapy (HBOT) and integrative wellness services, is excited to announce the opening of its newest clinic in Jacksonville, NC—a region defined by military service, resilience, and a...

Cortisol Levels Too High? Lower Them Naturally
In today’s fast-paced world, stress has become a constant companion for many people. Deadlines, notifications, traffic, poor sleep, and even our dietary habits all contribute to a steady rise in stress—and with it, rising cortisol levels. While some stress is normal...